[10000印刷√] T1/2 For First Order Reaction Is 141811-T1/2 For First Order Reaction Is 10 Minutes

A First Order Reaction Takes 10 Minutes For 25 Decomposition Calculate T1 2 For The Reaction Chemistry Chemical Kinetics Meritnation Com
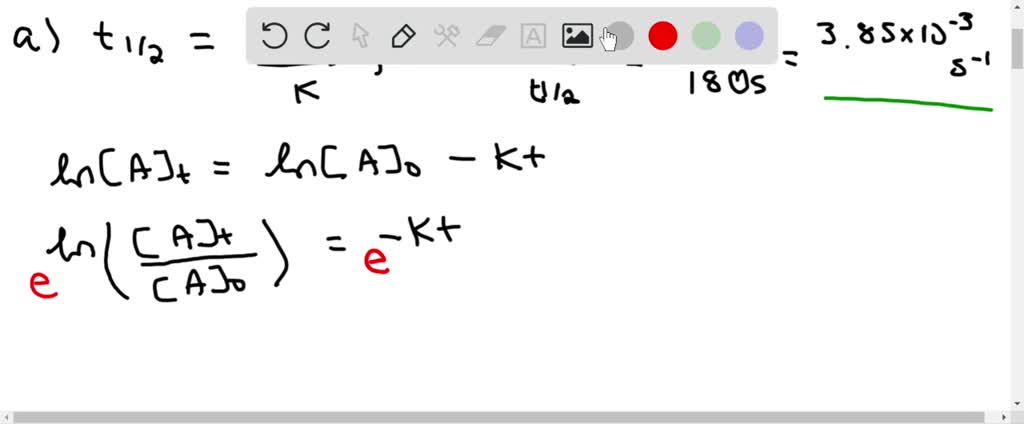
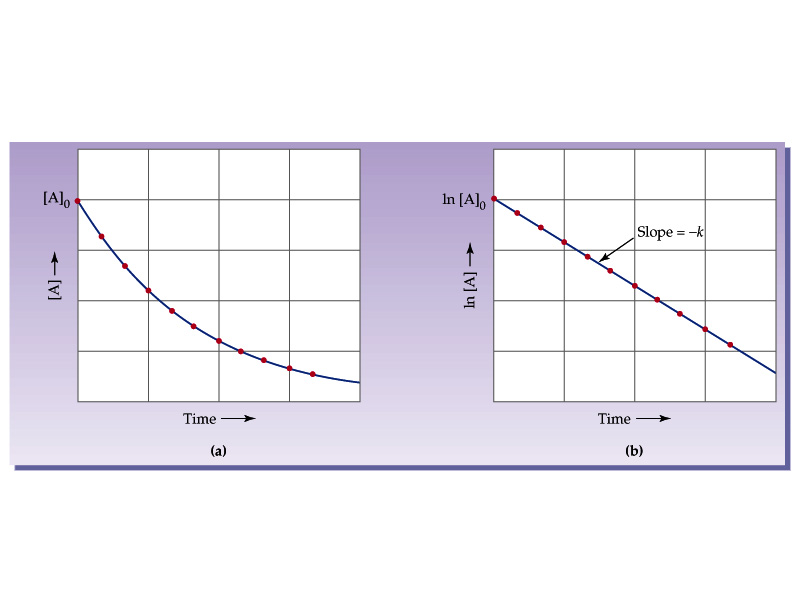
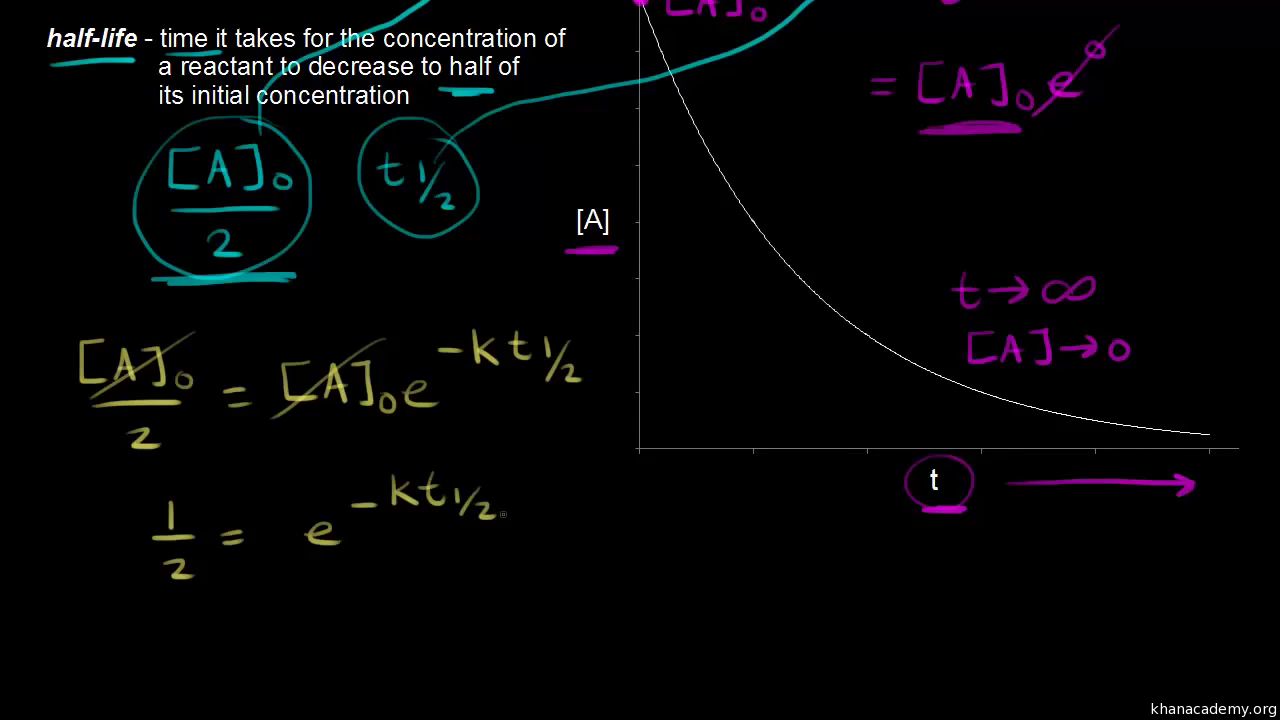
t1/2 for first order reaction is (a) 06/k (b) 0693/k (c) 06/k (d) 010/kA first order reaction takes 40 min for 30% decomposition Calculate t1/2 Solution For a first order reaction, Therefore, t1/2 of the decomposition reaction is = 777 min (approximately) Suggest
T1/2 for first order reaction is 10 minutes
T1/2 for first order reaction is 10 minutes-Halflife equation for firstorder reactions t1/2=0693k where t1/2 is the halflife in seconds (s), and k is the rate constant in inverse seconds (s−1) AT1 / 2 for a first order reaction is 10 min Starting with 10 M, the rate after min is Q t 1/2 for a first order reaction is 10 min Starting with 10 M , the rate after min is

Second Order Reaction Definition And Derivation For Rate Law And Half Life
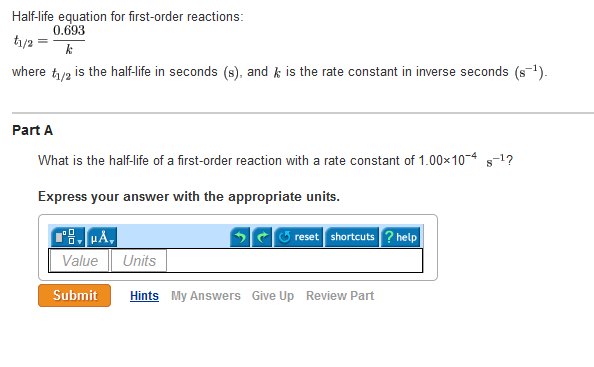
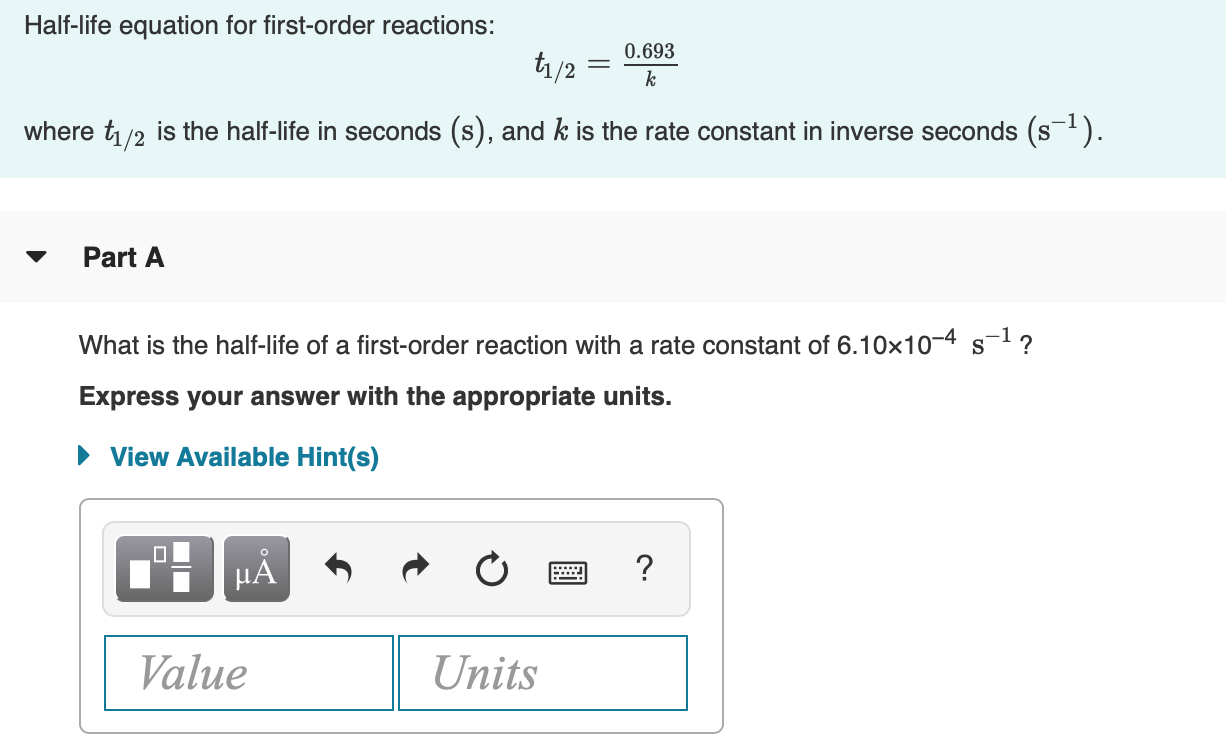
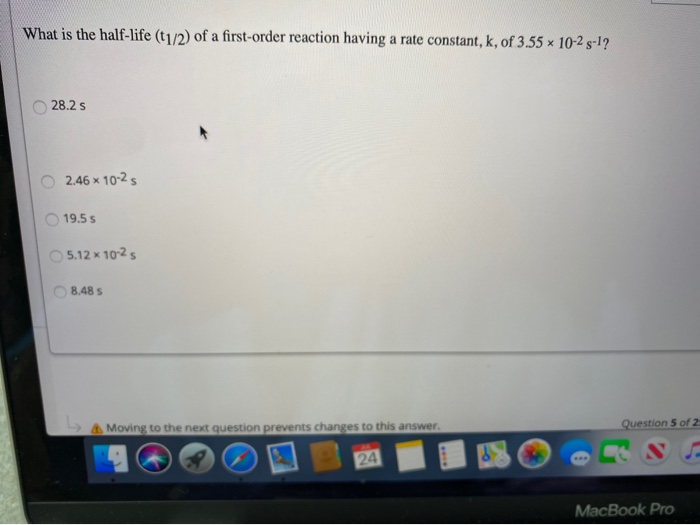
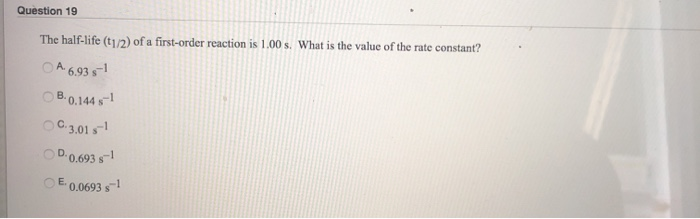
Halflife equation for firstorder reactions t1/2=0693k where t1/2 is the halflife in seconds (s), and k is the rate constant in inverse seconds (s−1) Part A What is the halflife of a firstorder Formula for t1⁄2 of First order reaction, t1⁄2 = 0693 k ∴ k = 0693 t1⁄2 By putting the value t1⁄2 in the equation k = 0693x60 k = min1Halflife (t1/2) of first order reaction is ← Prev Question Next Question → 0 votes 44 views asked in Chemistry by KushbooSahu (375k points) Halflife (t 1/2) of first order
T 1/2 of first order reaction is 10 min Starting with 10 molL −1, rate after min is A molL −1min −1 B ×25 molL −1min −1 C ×5 molL −1min −1 D ×10 molL −1min −1For a first order reaction, t1/2 = k0693 t1/2 ∝ k1 Halflife is independent of initial concentration of reactants for first order reaction 95 Likes Didn't understand the solution?The t1/2 of first order reaction is Tardigrade Jamia 12 The t1/2 of first order reaction is (A) dependent of initial concentration (B) directly proportional to initial concentration indirect
T1/2 for first order reaction is 10 minutesのギャラリー
各画像をクリックすると、ダウンロードまたは拡大表示できます
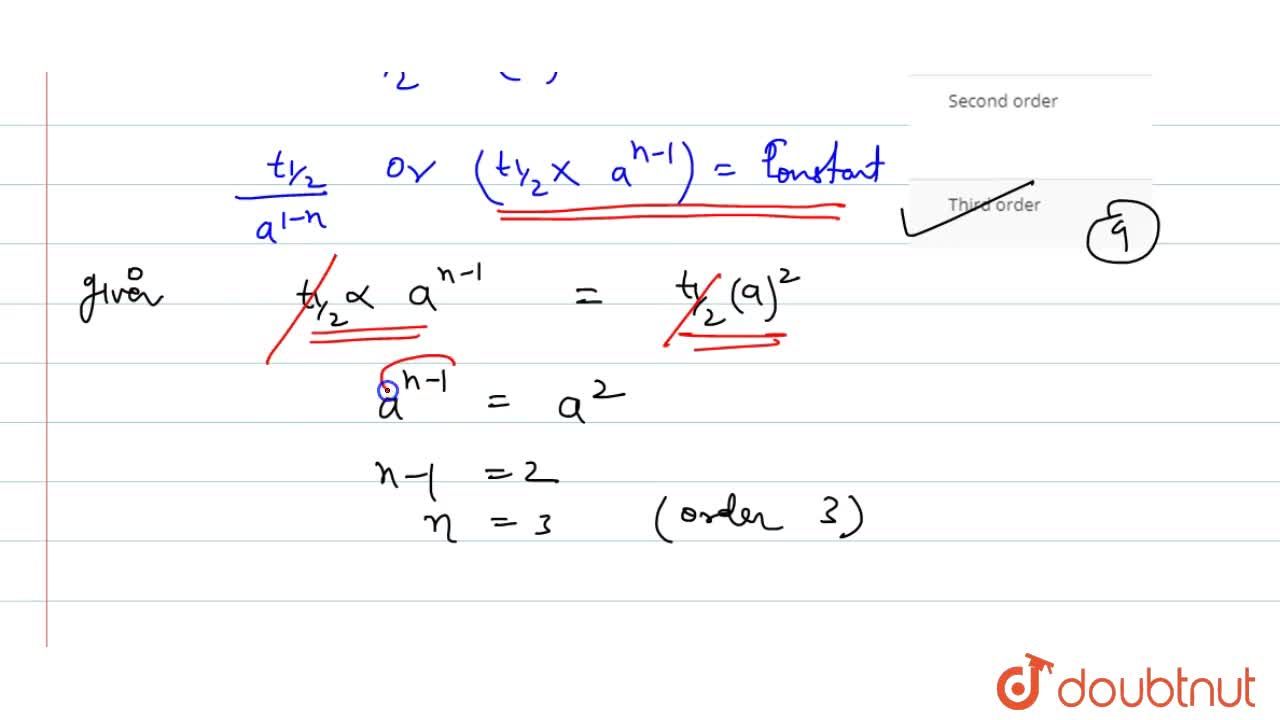
 If The Half Life Period Of A Reaction Is 0 693 K Its Order Will Be |  If The Half Life Period Of A Reaction Is 0 693 K Its Order Will Be |  If The Half Life Period Of A Reaction Is 0 693 K Its Order Will Be |
If The Half Life Period Of A Reaction Is 0 693 K Its Order Will Be | 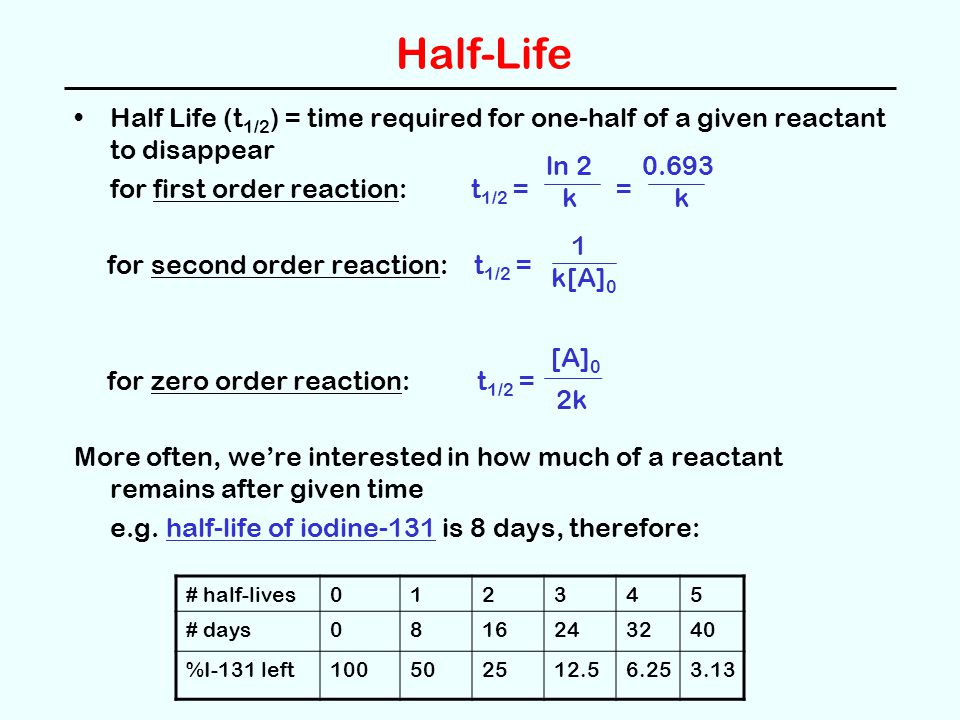 If The Half Life Period Of A Reaction Is 0 693 K Its Order Will Be | 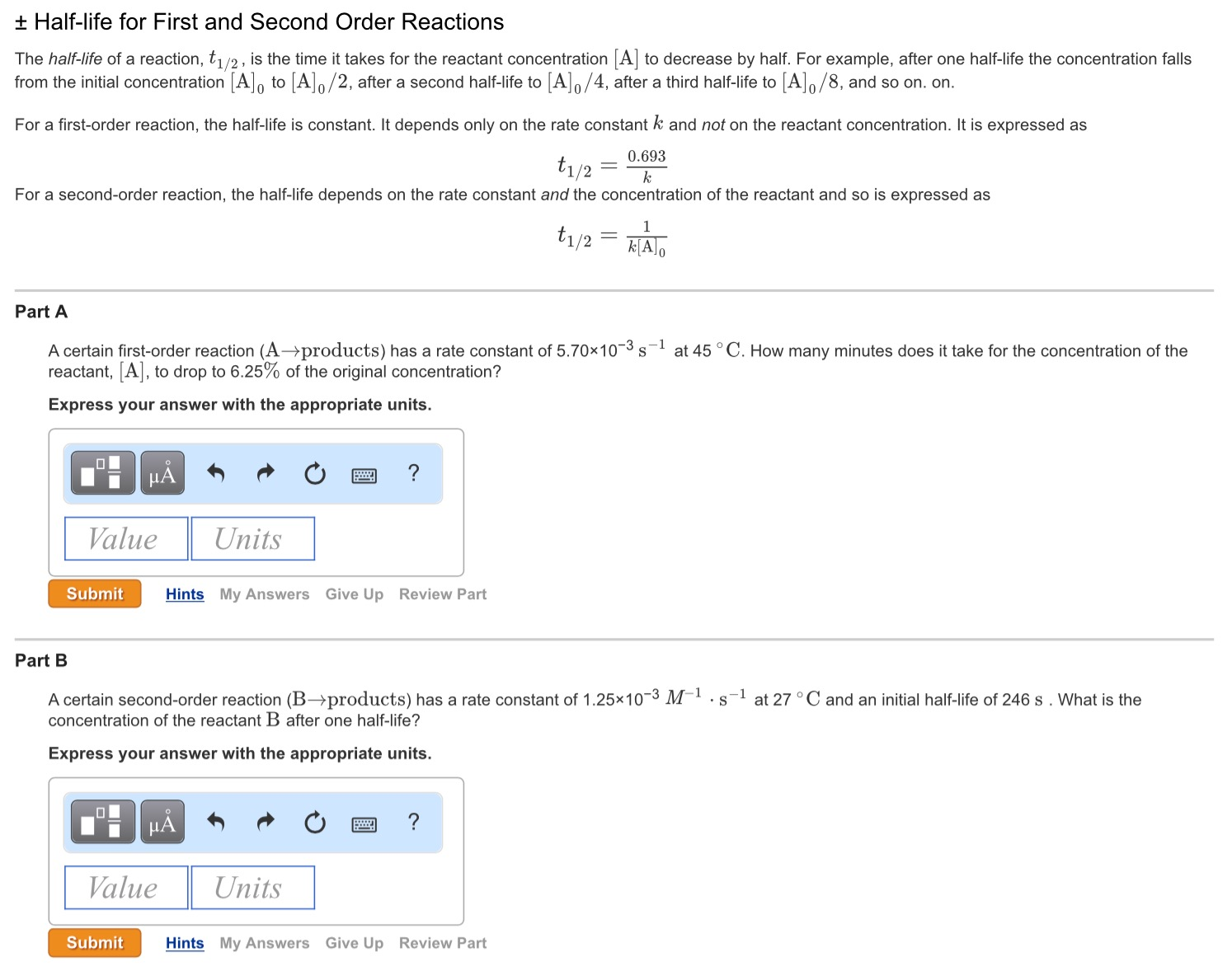 If The Half Life Period Of A Reaction Is 0 693 K Its Order Will Be |
If The Half Life Period Of A Reaction Is 0 693 K Its Order Will Be |  If The Half Life Period Of A Reaction Is 0 693 K Its Order Will Be |  If The Half Life Period Of A Reaction Is 0 693 K Its Order Will Be |
 If The Half Life Period Of A Reaction Is 0 693 K Its Order Will Be |  If The Half Life Period Of A Reaction Is 0 693 K Its Order Will Be | If The Half Life Period Of A Reaction Is 0 693 K Its Order Will Be |
If The Half Life Period Of A Reaction Is 0 693 K Its Order Will Be | 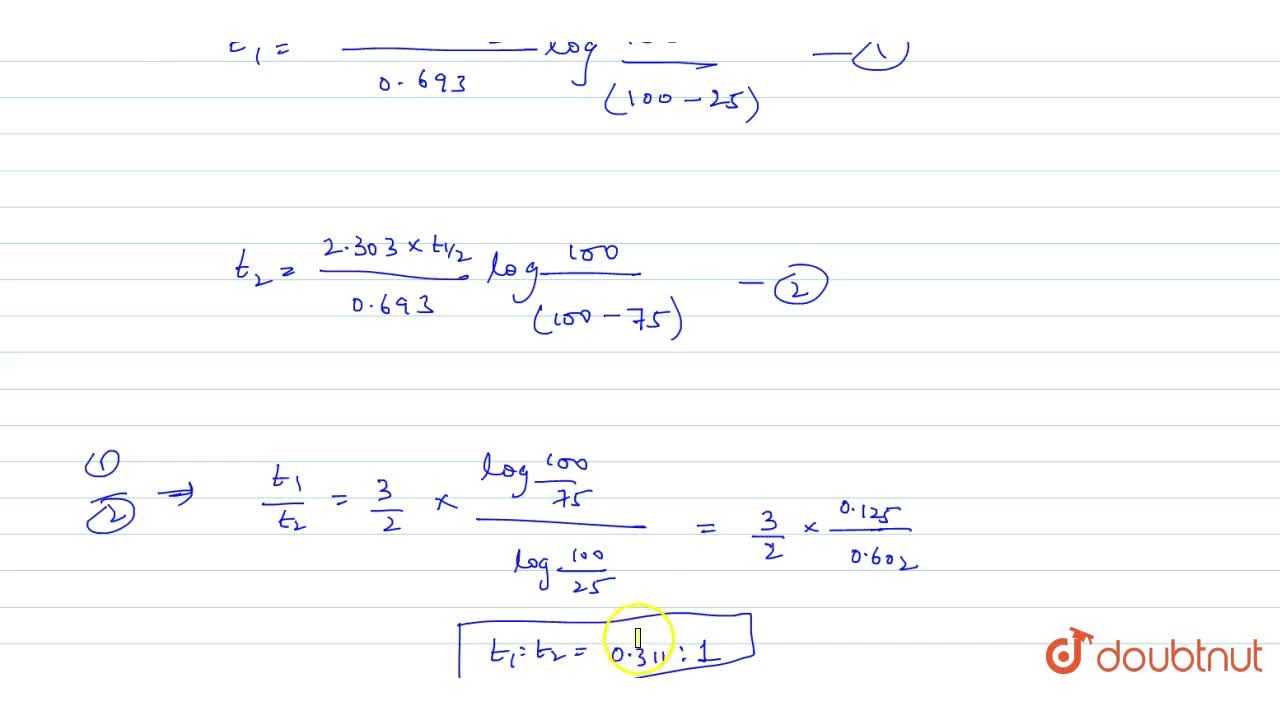 If The Half Life Period Of A Reaction Is 0 693 K Its Order Will Be | 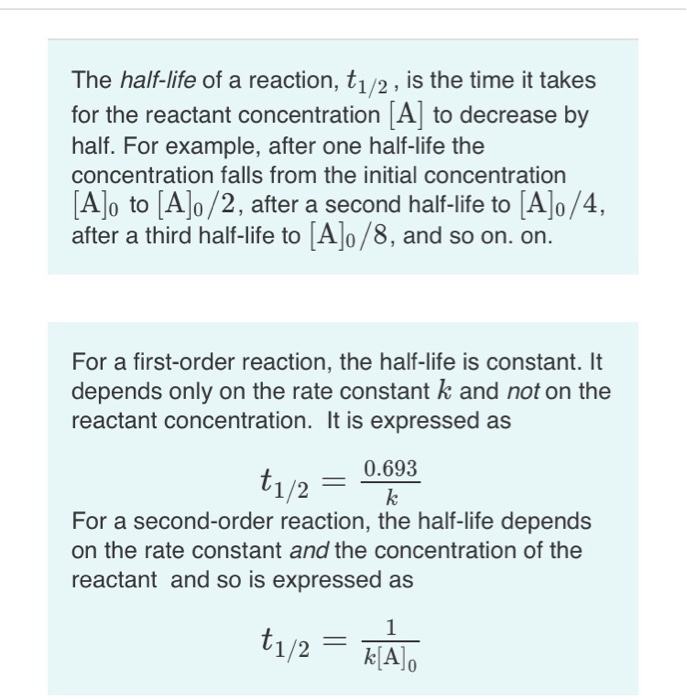 If The Half Life Period Of A Reaction Is 0 693 K Its Order Will Be |
 If The Half Life Period Of A Reaction Is 0 693 K Its Order Will Be |  If The Half Life Period Of A Reaction Is 0 693 K Its Order Will Be |  If The Half Life Period Of A Reaction Is 0 693 K Its Order Will Be |
 If The Half Life Period Of A Reaction Is 0 693 K Its Order Will Be | 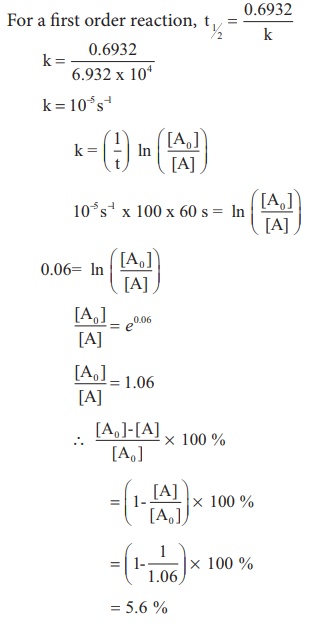 If The Half Life Period Of A Reaction Is 0 693 K Its Order Will Be |  If The Half Life Period Of A Reaction Is 0 693 K Its Order Will Be |
 If The Half Life Period Of A Reaction Is 0 693 K Its Order Will Be | 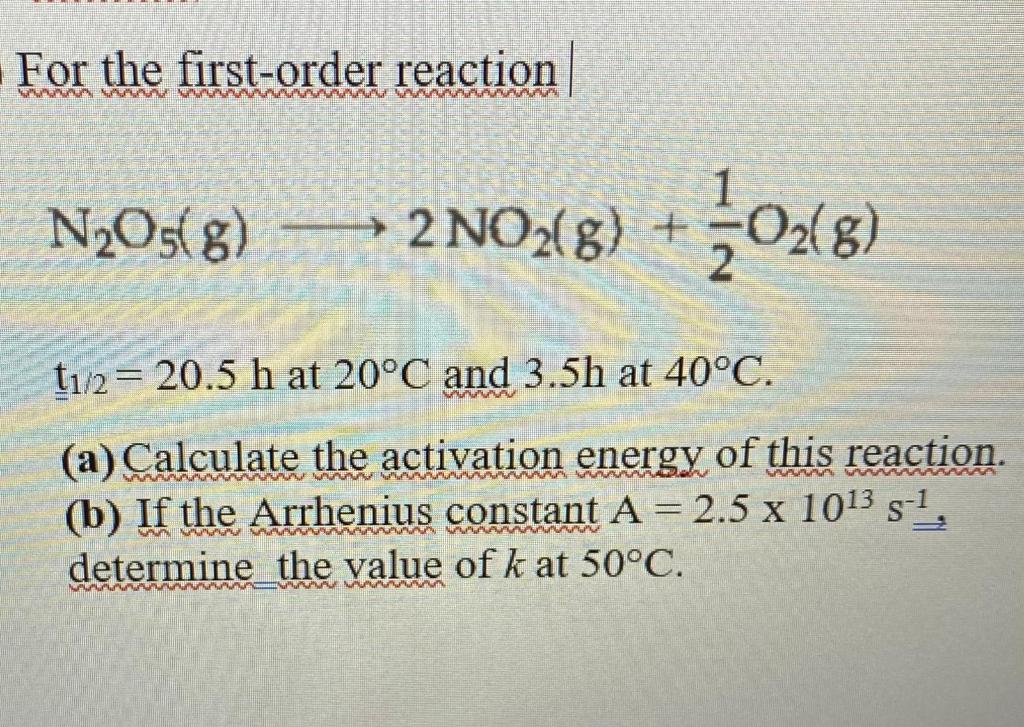 If The Half Life Period Of A Reaction Is 0 693 K Its Order Will Be |  If The Half Life Period Of A Reaction Is 0 693 K Its Order Will Be |
If The Half Life Period Of A Reaction Is 0 693 K Its Order Will Be |  If The Half Life Period Of A Reaction Is 0 693 K Its Order Will Be |  If The Half Life Period Of A Reaction Is 0 693 K Its Order Will Be |
 If The Half Life Period Of A Reaction Is 0 693 K Its Order Will Be |  If The Half Life Period Of A Reaction Is 0 693 K Its Order Will Be |  If The Half Life Period Of A Reaction Is 0 693 K Its Order Will Be |
 If The Half Life Period Of A Reaction Is 0 693 K Its Order Will Be | If The Half Life Period Of A Reaction Is 0 693 K Its Order Will Be | 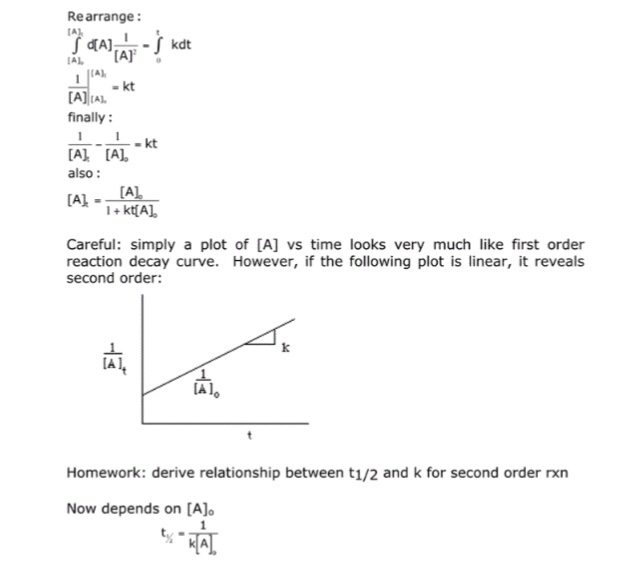 If The Half Life Period Of A Reaction Is 0 693 K Its Order Will Be |
 If The Half Life Period Of A Reaction Is 0 693 K Its Order Will Be | If The Half Life Period Of A Reaction Is 0 693 K Its Order Will Be | 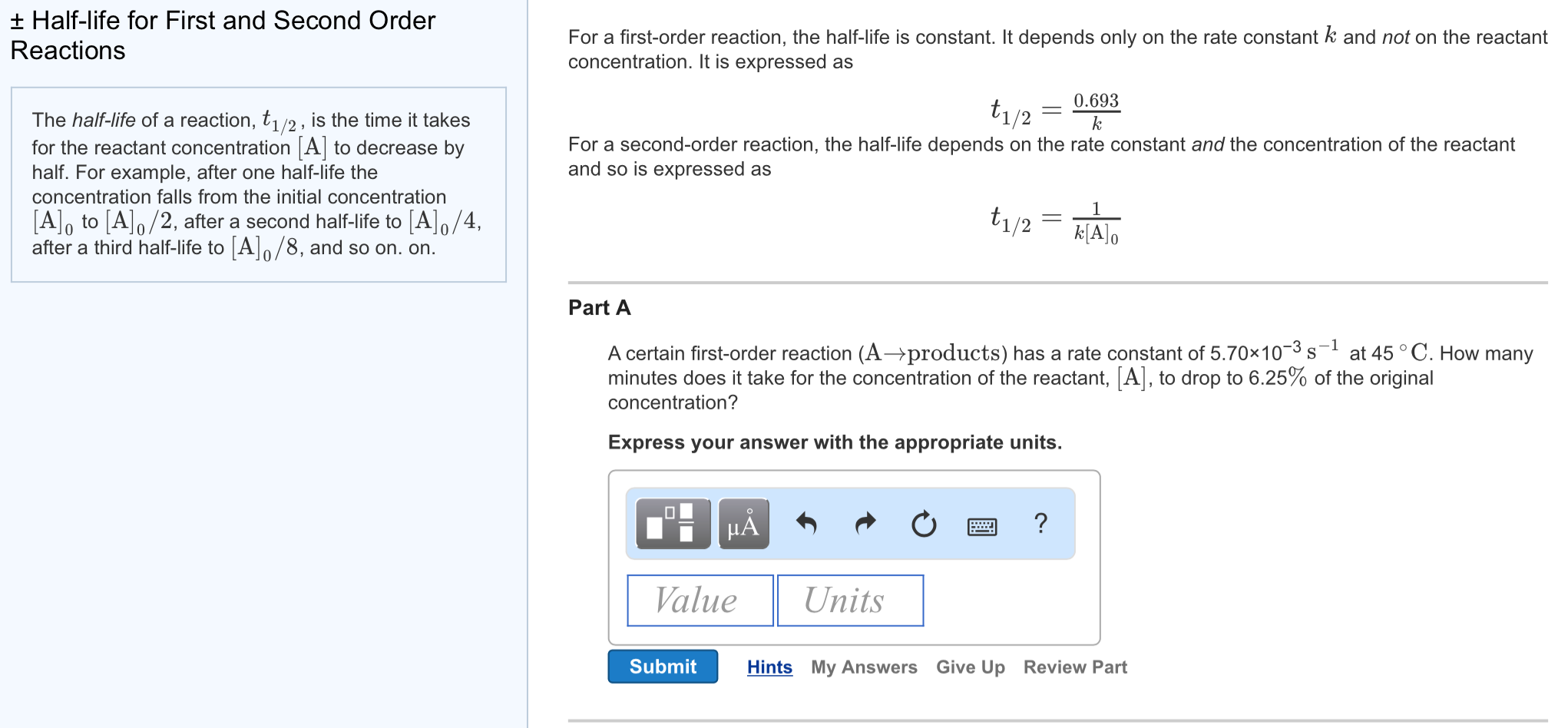 If The Half Life Period Of A Reaction Is 0 693 K Its Order Will Be |
 If The Half Life Period Of A Reaction Is 0 693 K Its Order Will Be | 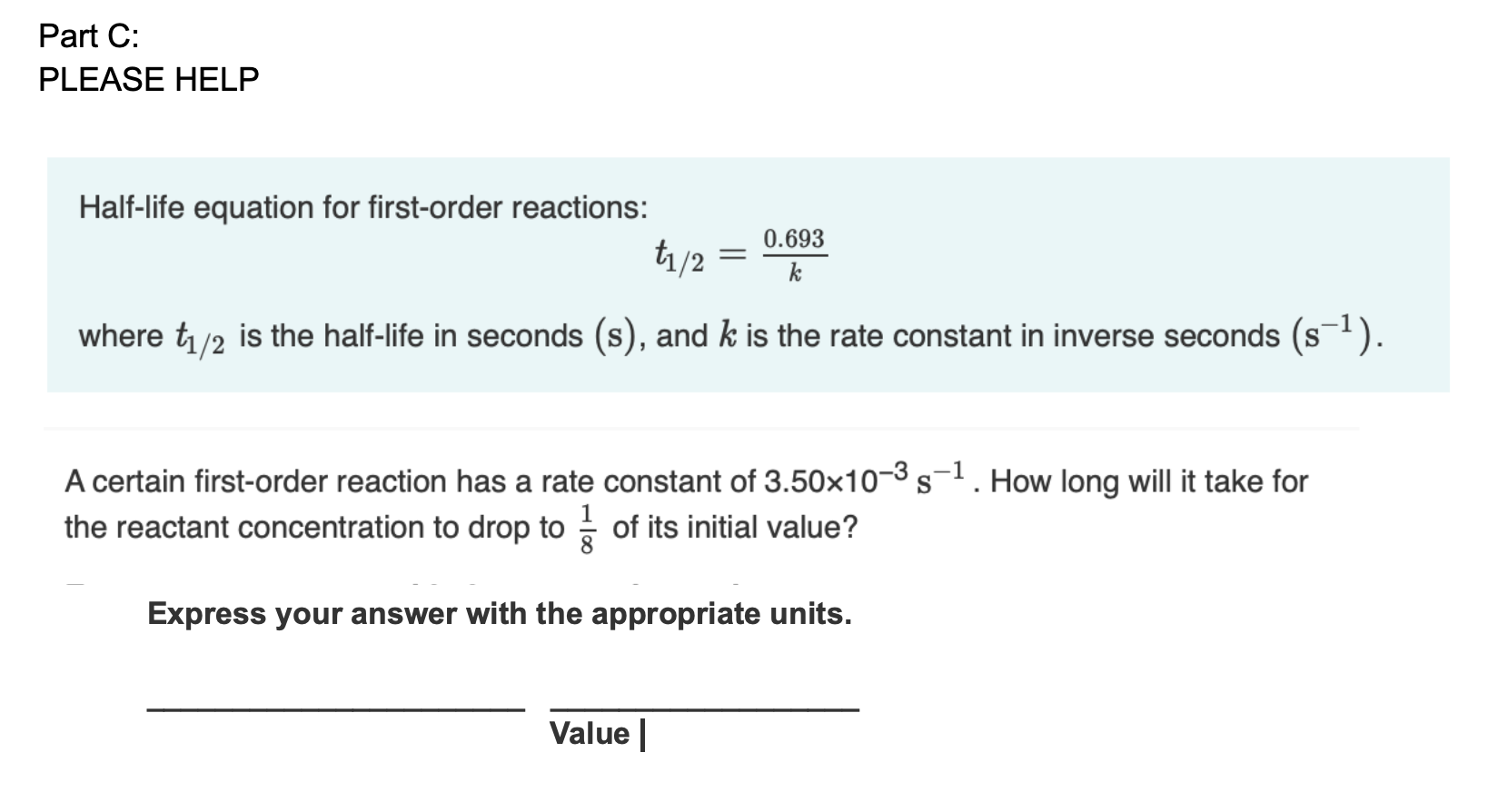 If The Half Life Period Of A Reaction Is 0 693 K Its Order Will Be |  If The Half Life Period Of A Reaction Is 0 693 K Its Order Will Be |
 If The Half Life Period Of A Reaction Is 0 693 K Its Order Will Be | 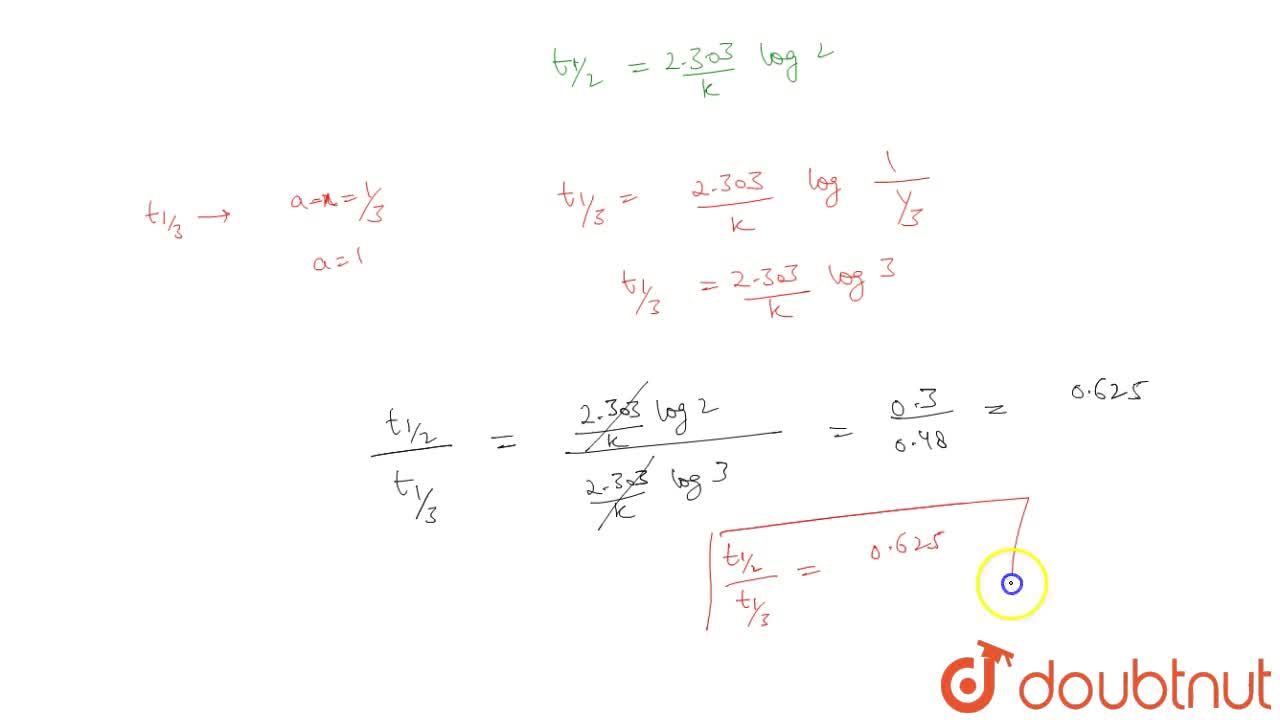 If The Half Life Period Of A Reaction Is 0 693 K Its Order Will Be | 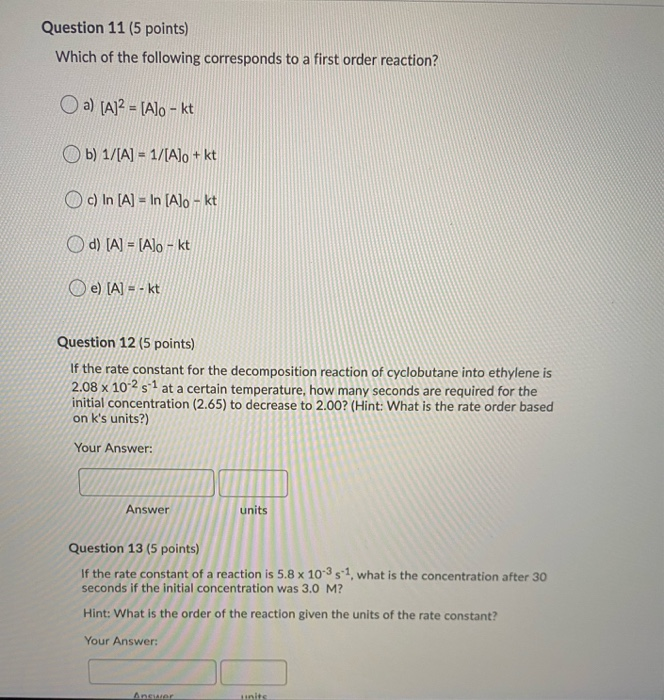 If The Half Life Period Of A Reaction Is 0 693 K Its Order Will Be |
 If The Half Life Period Of A Reaction Is 0 693 K Its Order Will Be |  If The Half Life Period Of A Reaction Is 0 693 K Its Order Will Be |  If The Half Life Period Of A Reaction Is 0 693 K Its Order Will Be |
 If The Half Life Period Of A Reaction Is 0 693 K Its Order Will Be |  If The Half Life Period Of A Reaction Is 0 693 K Its Order Will Be |  If The Half Life Period Of A Reaction Is 0 693 K Its Order Will Be |
 If The Half Life Period Of A Reaction Is 0 693 K Its Order Will Be | 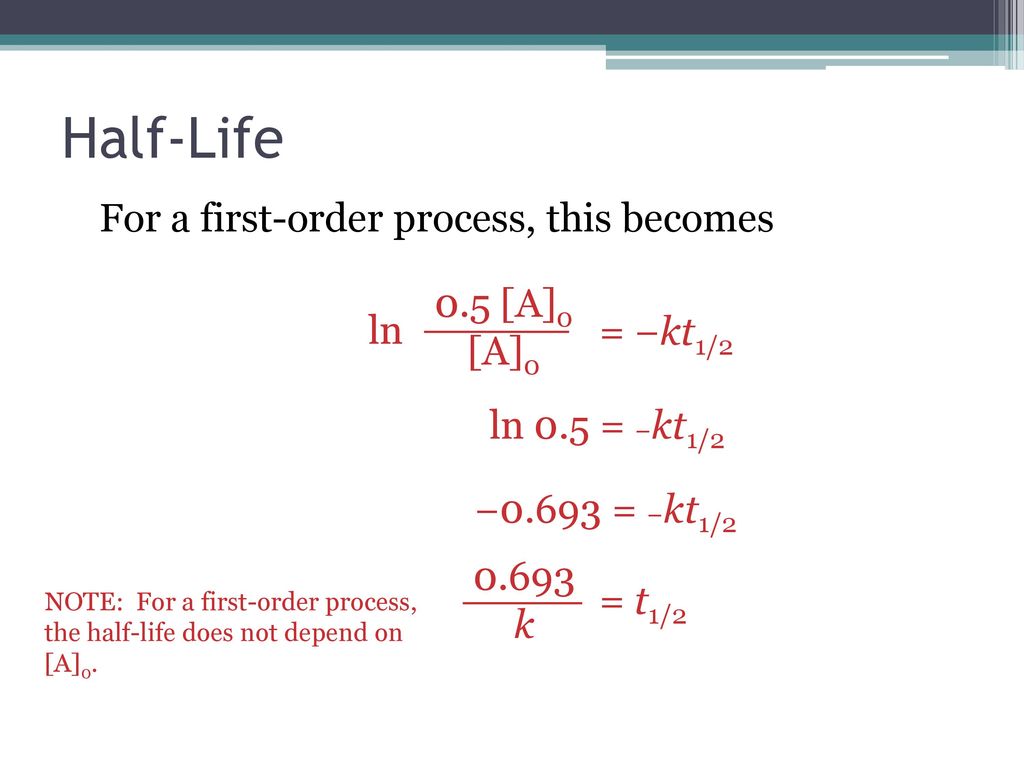 If The Half Life Period Of A Reaction Is 0 693 K Its Order Will Be |  If The Half Life Period Of A Reaction Is 0 693 K Its Order Will Be |
 If The Half Life Period Of A Reaction Is 0 693 K Its Order Will Be | If The Half Life Period Of A Reaction Is 0 693 K Its Order Will Be |  If The Half Life Period Of A Reaction Is 0 693 K Its Order Will Be |
 If The Half Life Period Of A Reaction Is 0 693 K Its Order Will Be |  If The Half Life Period Of A Reaction Is 0 693 K Its Order Will Be |  If The Half Life Period Of A Reaction Is 0 693 K Its Order Will Be |
 If The Half Life Period Of A Reaction Is 0 693 K Its Order Will Be |  If The Half Life Period Of A Reaction Is 0 693 K Its Order Will Be |  If The Half Life Period Of A Reaction Is 0 693 K Its Order Will Be |
If The Half Life Period Of A Reaction Is 0 693 K Its Order Will Be | 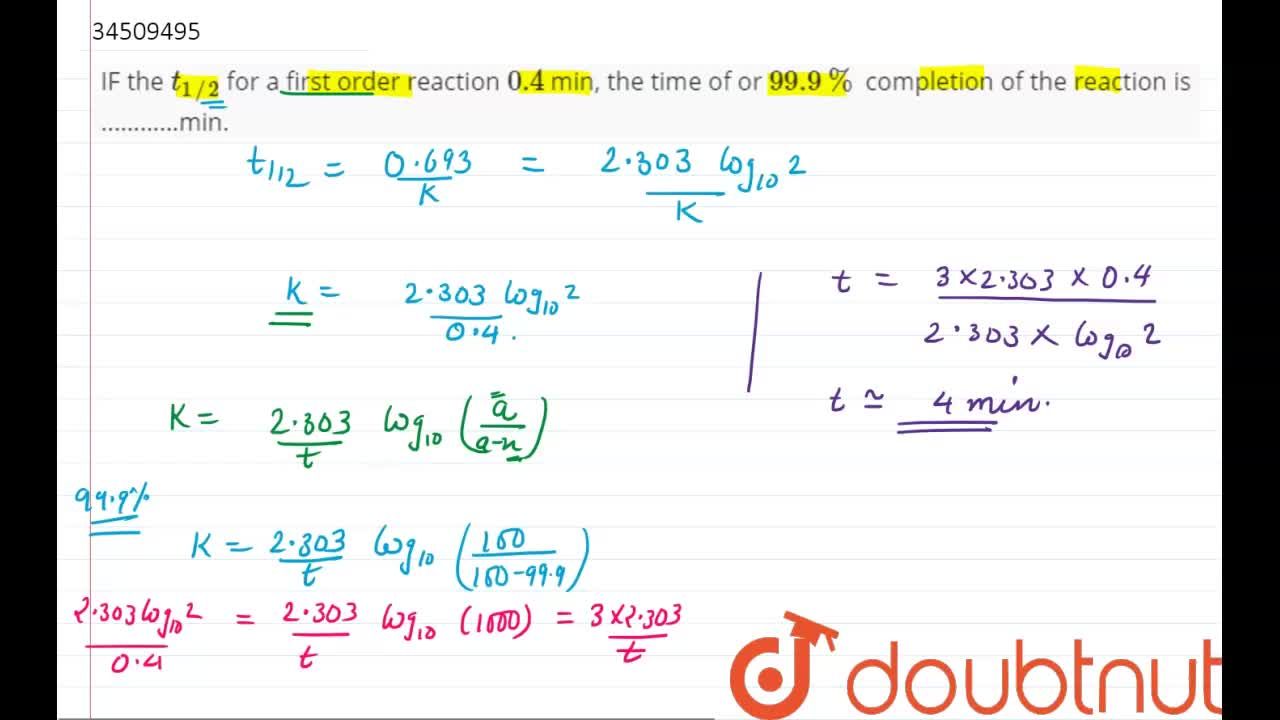 If The Half Life Period Of A Reaction Is 0 693 K Its Order Will Be | 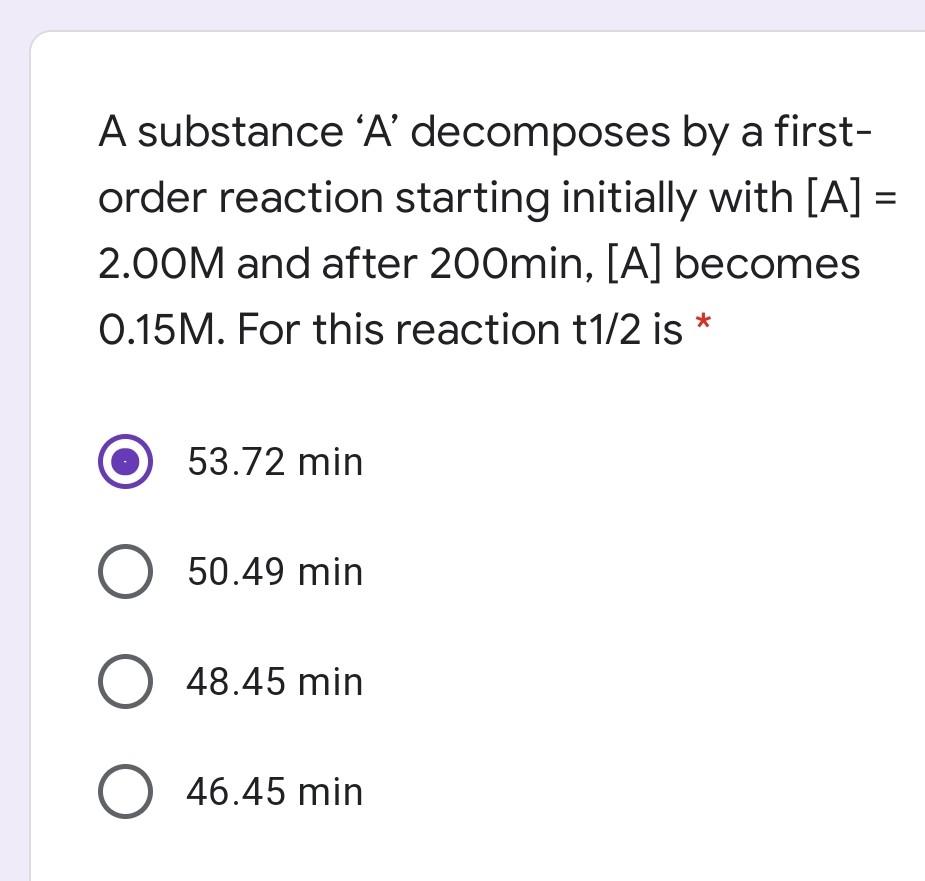 If The Half Life Period Of A Reaction Is 0 693 K Its Order Will Be |
 If The Half Life Period Of A Reaction Is 0 693 K Its Order Will Be |  If The Half Life Period Of A Reaction Is 0 693 K Its Order Will Be |  If The Half Life Period Of A Reaction Is 0 693 K Its Order Will Be |
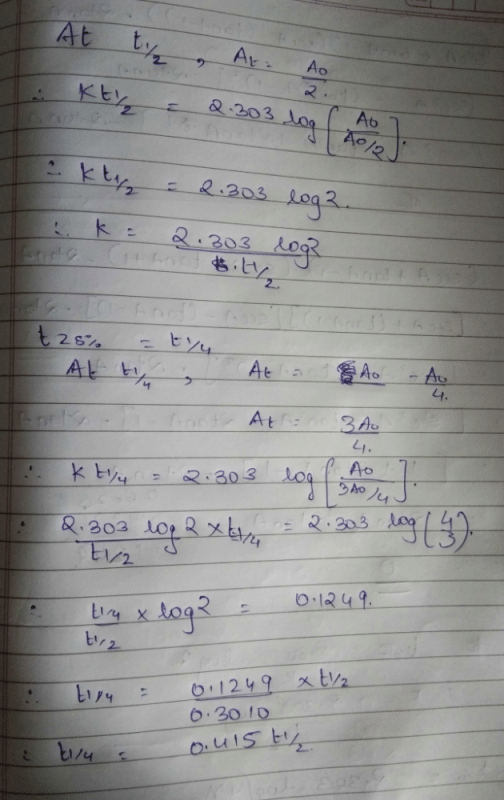 If The Half Life Period Of A Reaction Is 0 693 K Its Order Will Be |  If The Half Life Period Of A Reaction Is 0 693 K Its Order Will Be |  If The Half Life Period Of A Reaction Is 0 693 K Its Order Will Be |
If The Half Life Period Of A Reaction Is 0 693 K Its Order Will Be | 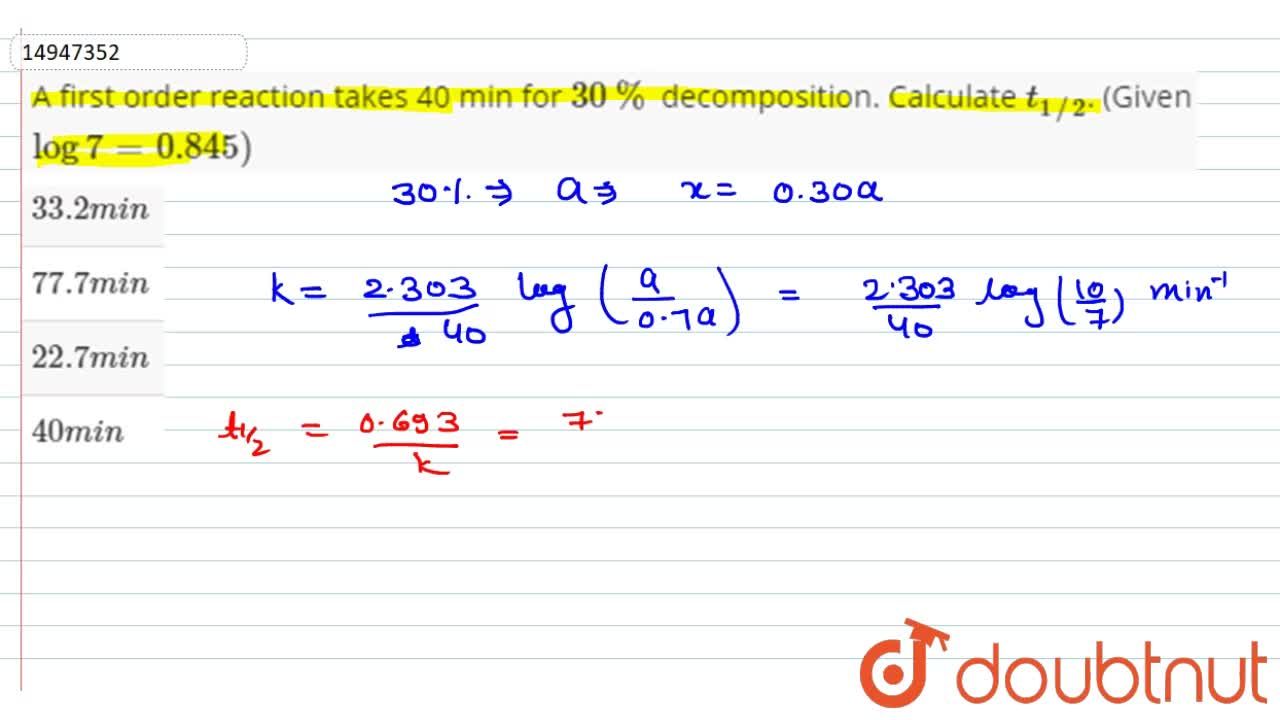 If The Half Life Period Of A Reaction Is 0 693 K Its Order Will Be |  If The Half Life Period Of A Reaction Is 0 693 K Its Order Will Be |
 If The Half Life Period Of A Reaction Is 0 693 K Its Order Will Be |  If The Half Life Period Of A Reaction Is 0 693 K Its Order Will Be |  If The Half Life Period Of A Reaction Is 0 693 K Its Order Will Be |
 If The Half Life Period Of A Reaction Is 0 693 K Its Order Will Be |  If The Half Life Period Of A Reaction Is 0 693 K Its Order Will Be | If The Half Life Period Of A Reaction Is 0 693 K Its Order Will Be |
 If The Half Life Period Of A Reaction Is 0 693 K Its Order Will Be |  If The Half Life Period Of A Reaction Is 0 693 K Its Order Will Be | 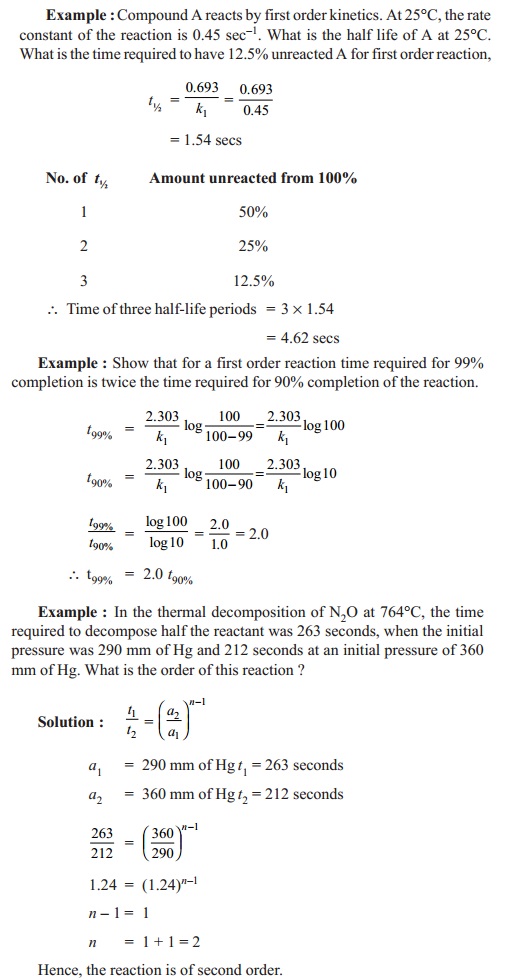 If The Half Life Period Of A Reaction Is 0 693 K Its Order Will Be |
 If The Half Life Period Of A Reaction Is 0 693 K Its Order Will Be | 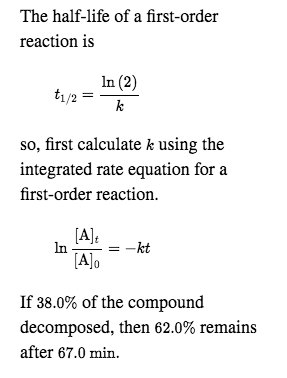 If The Half Life Period Of A Reaction Is 0 693 K Its Order Will Be | 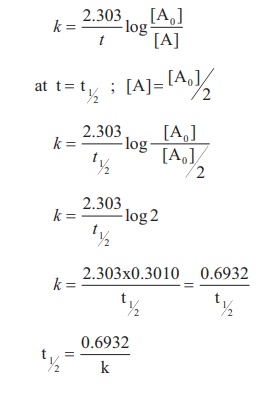 If The Half Life Period Of A Reaction Is 0 693 K Its Order Will Be |
 If The Half Life Period Of A Reaction Is 0 693 K Its Order Will Be |  If The Half Life Period Of A Reaction Is 0 693 K Its Order Will Be |  If The Half Life Period Of A Reaction Is 0 693 K Its Order Will Be |
 If The Half Life Period Of A Reaction Is 0 693 K Its Order Will Be | 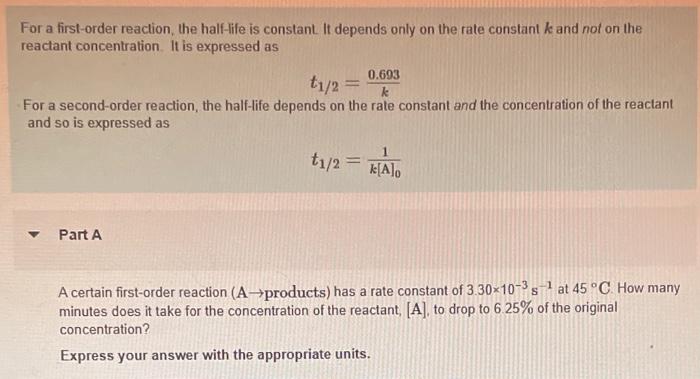 If The Half Life Period Of A Reaction Is 0 693 K Its Order Will Be |  If The Half Life Period Of A Reaction Is 0 693 K Its Order Will Be |
 If The Half Life Period Of A Reaction Is 0 693 K Its Order Will Be | If The Half Life Period Of A Reaction Is 0 693 K Its Order Will Be | 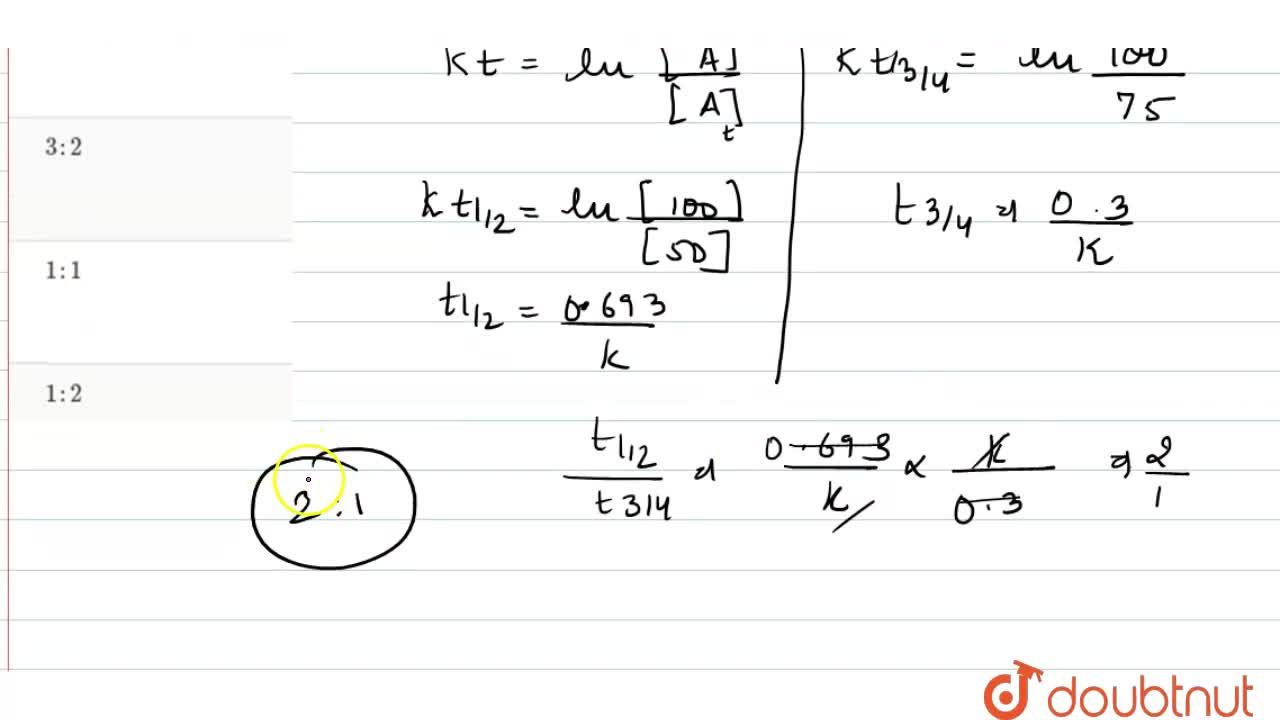 If The Half Life Period Of A Reaction Is 0 693 K Its Order Will Be |
 If The Half Life Period Of A Reaction Is 0 693 K Its Order Will Be |  If The Half Life Period Of A Reaction Is 0 693 K Its Order Will Be |  If The Half Life Period Of A Reaction Is 0 693 K Its Order Will Be |
If The Half Life Period Of A Reaction Is 0 693 K Its Order Will Be |  If The Half Life Period Of A Reaction Is 0 693 K Its Order Will Be | 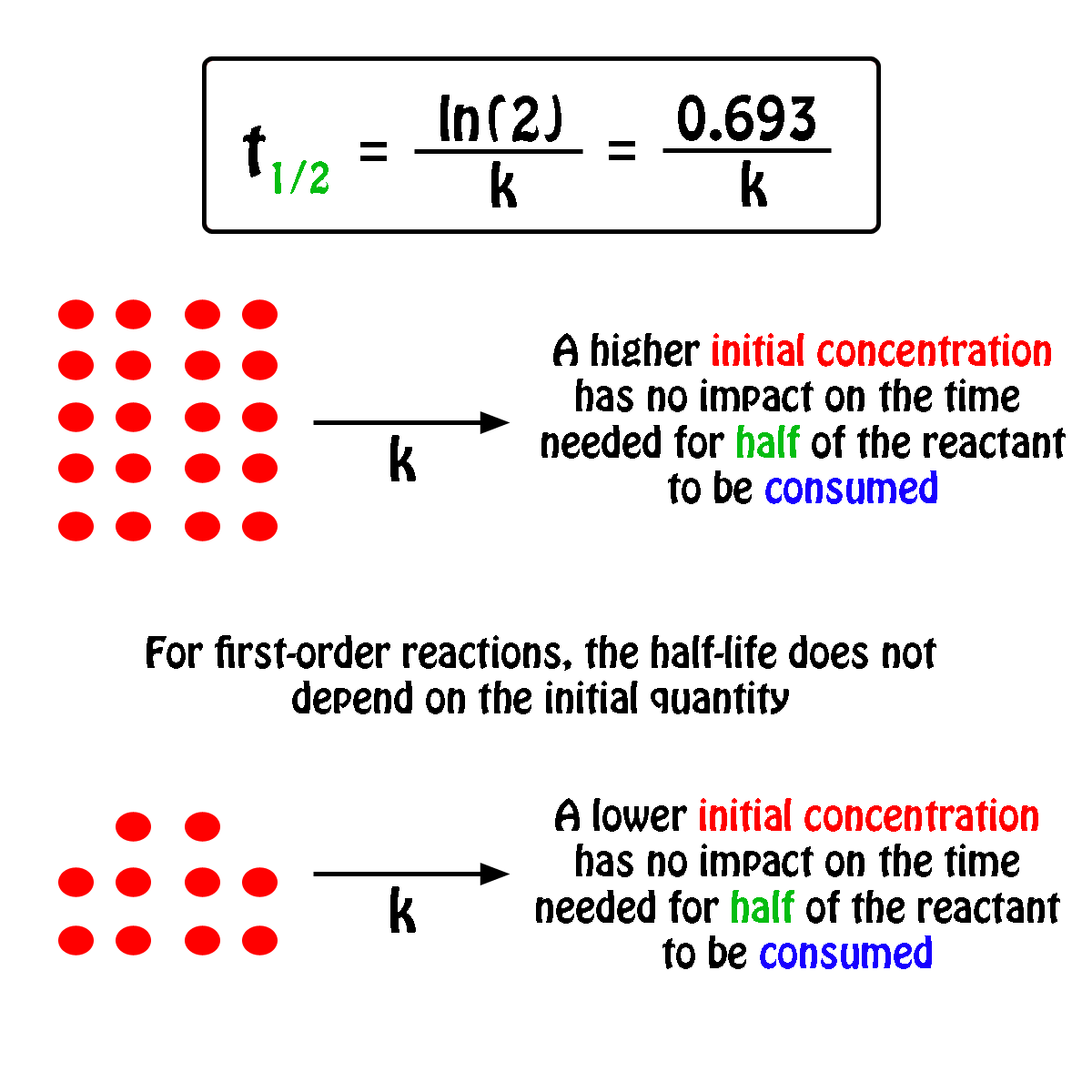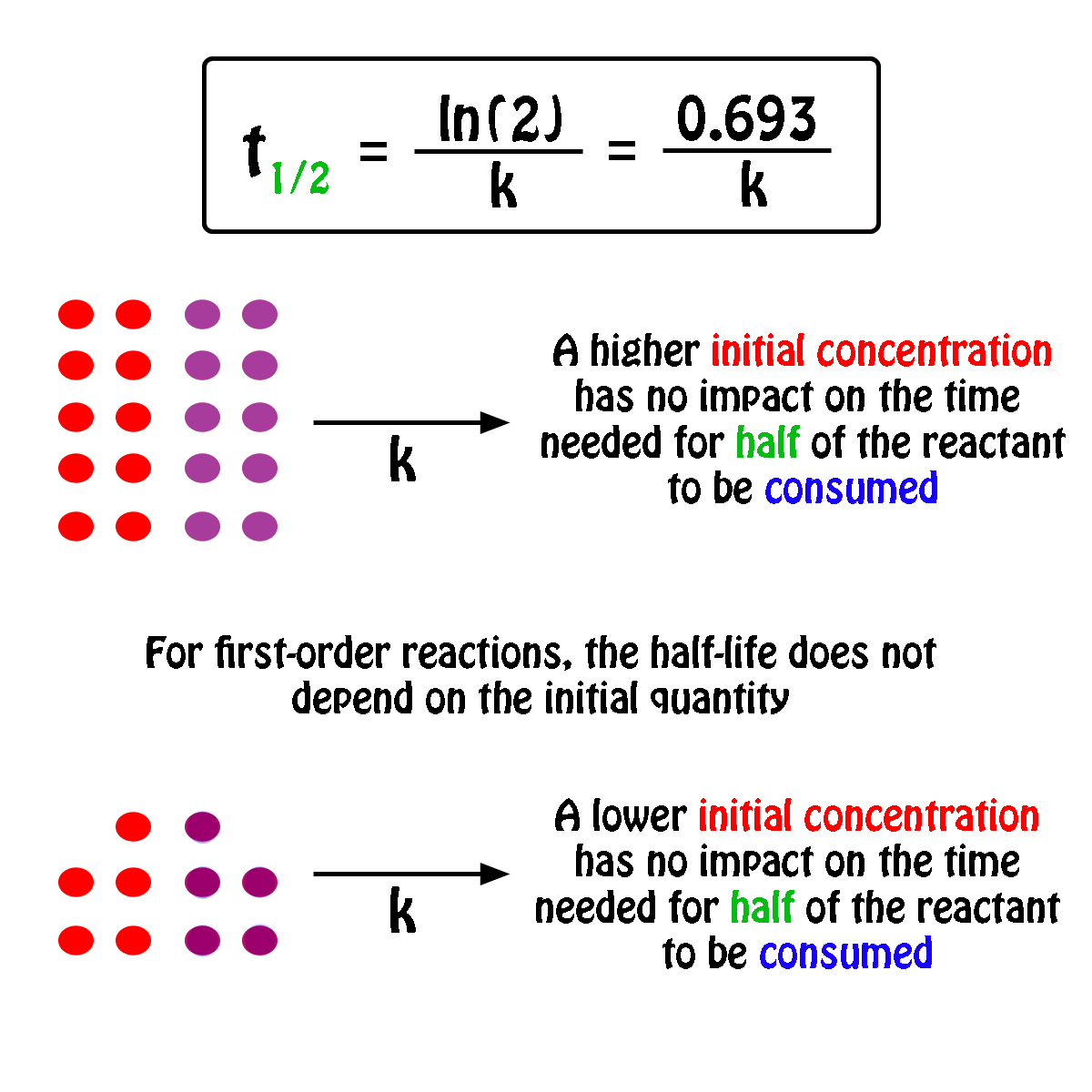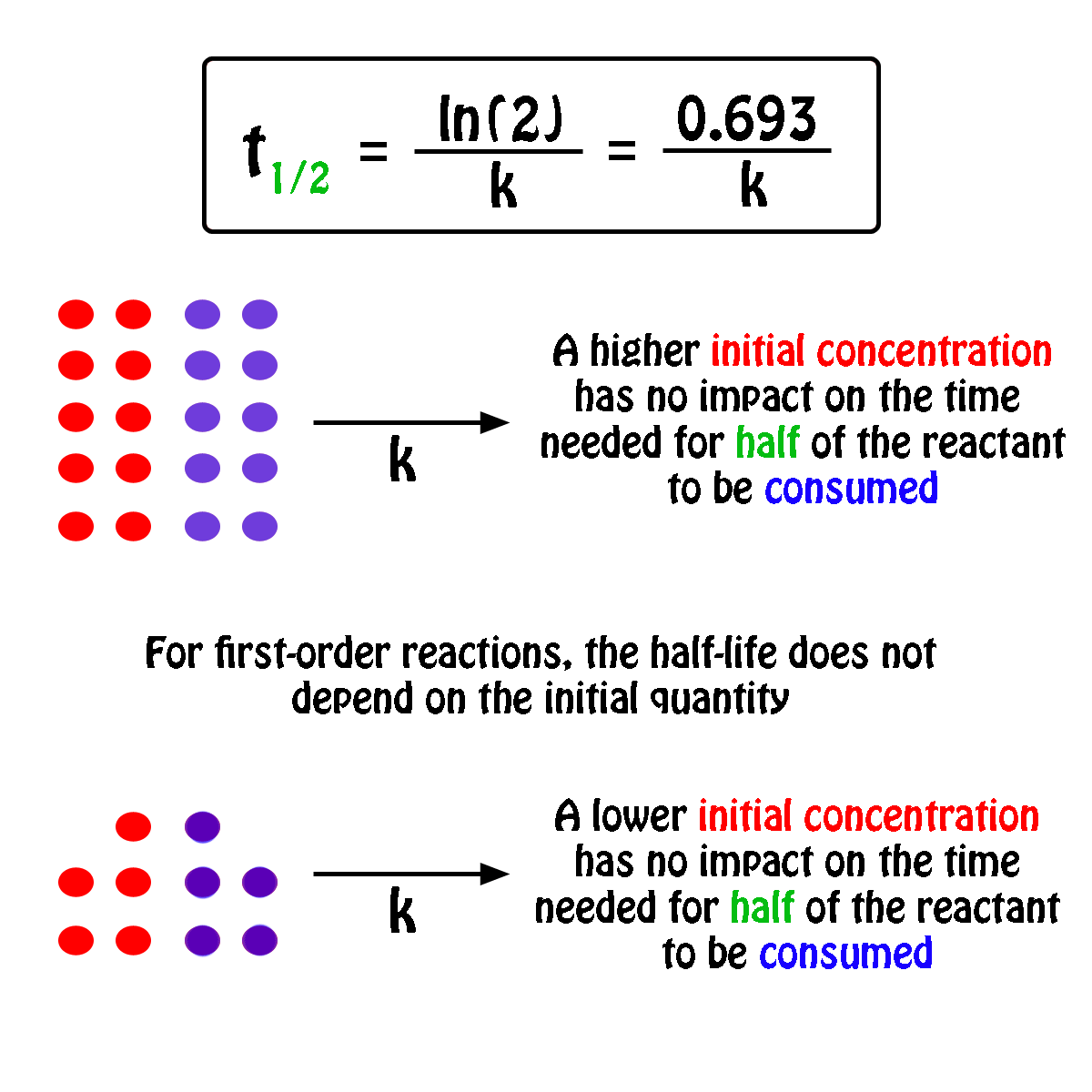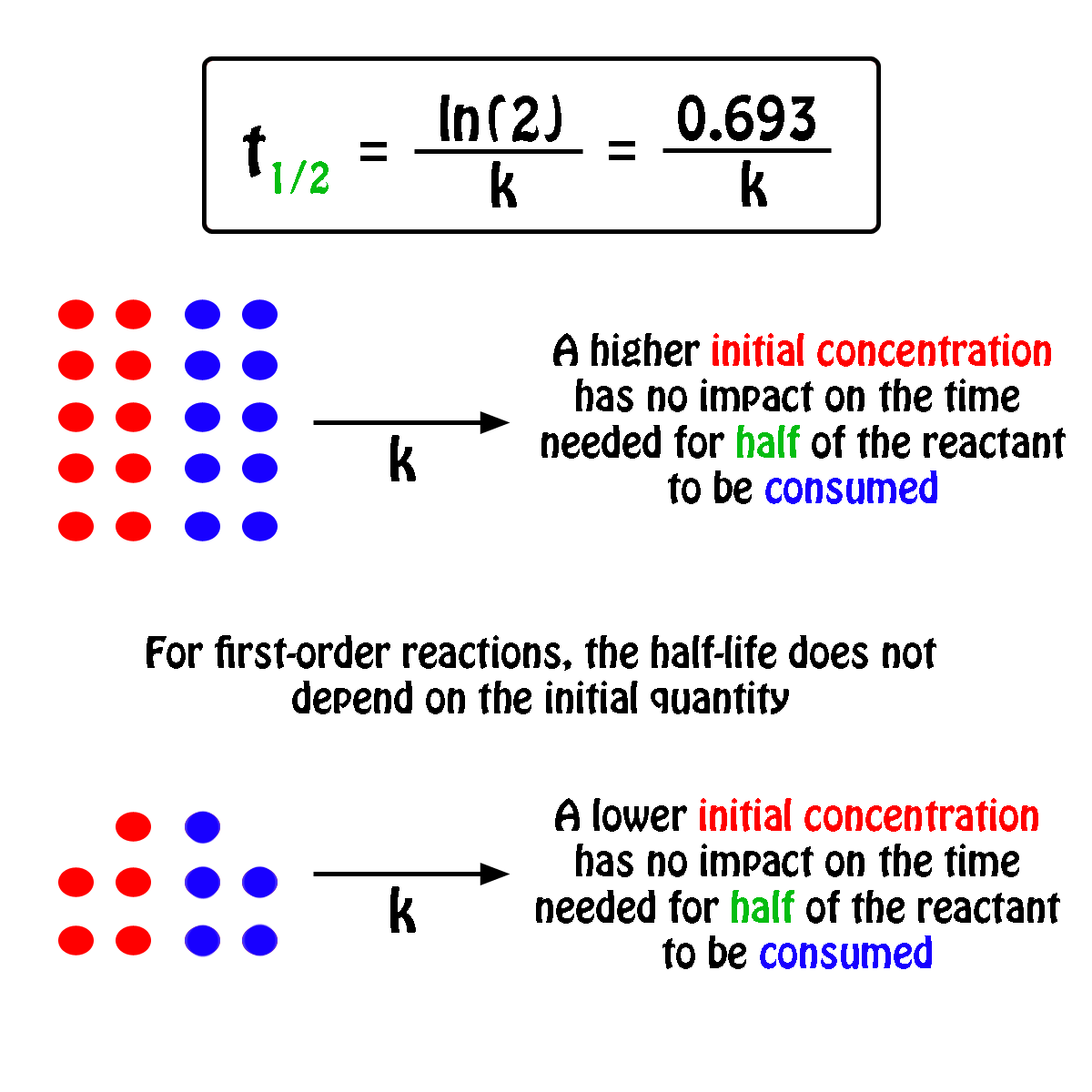 If The Half Life Period Of A Reaction Is 0 693 K Its Order Will Be |
 If The Half Life Period Of A Reaction Is 0 693 K Its Order Will Be | 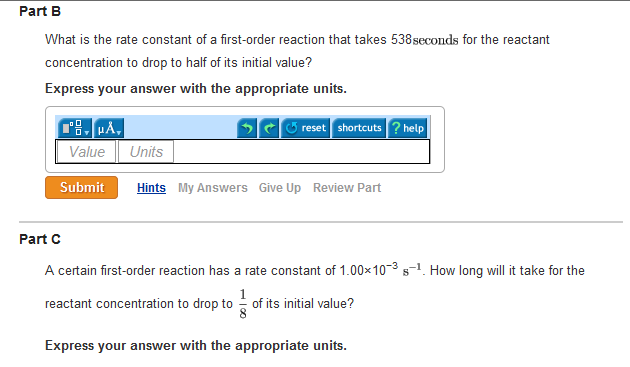 If The Half Life Period Of A Reaction Is 0 693 K Its Order Will Be | If The Half Life Period Of A Reaction Is 0 693 K Its Order Will Be |
 If The Half Life Period Of A Reaction Is 0 693 K Its Order Will Be |  If The Half Life Period Of A Reaction Is 0 693 K Its Order Will Be |
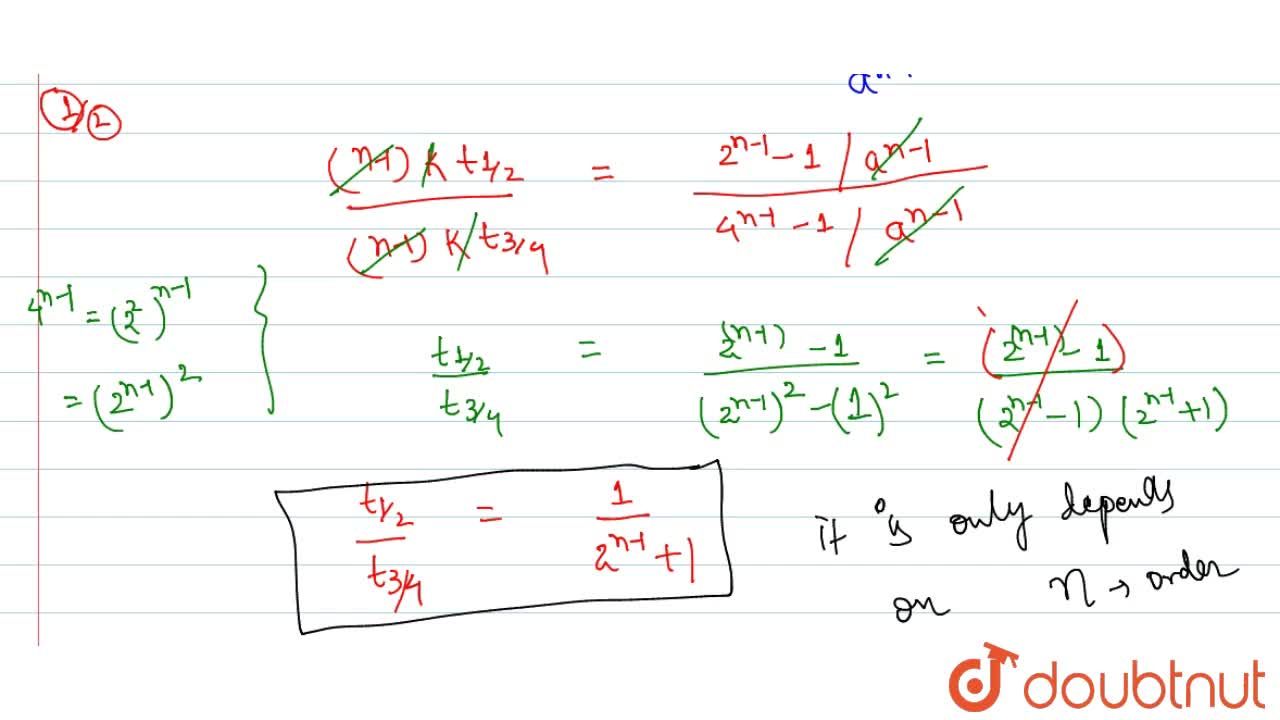
Ask a Tutor PracticeFor a first order reaction, t 1/2 is thrice the t 7/8 For a first order reaction, t 1/2 is independent of the initial concentration of reactants A Both Assertion and Reason are correct and Reason is the
Incoming Term: t1/2 for first order reaction is 10 minutes, t1/2 for first order reaction is, t1/2 for first order reaction is 14.26 minutes, t1/2 of first order reaction is 60 minutes, the value of t1/2 for first order reaction is, half life period t1/2) for first order reaction is, if t1/2 of first order reaction is 40 min, for first order reaction t1/2 is proportional to, the relation between t1/2 and k for first order reaction is, the ratio t7/8 t1/2 for the first order reaction is,
コメント
コメントを投稿